Discover how SR-TIGET scientists helped shape the science of peripheral immune tolerance, from the discovery of Treg and Tr1 cells to early gene therapy.

When the 2025 Nobel Prize in Physiology or Medicine was awarded to Shimon Sakaguchi, Mary Brunkow and Fred Ramsdell for the discovery and characterization of peripheral immune tolerance and regulatory T cells (Tregs), the scientific community celebrated one of the cornerstones of modern immunology. But this achievement honors not only three pioneers: it also reflects decades of international collaboration that have helped us understand how the immune system maintains its delicate balance between defense and self-control.
Among the key contributors to this story is the San Raffaele–Telethon Institute for Gene Therapy (SR-TIGET), which played a central role from the very beginning, thanks to the pioneering work of Maria Grazia Roncarolo and her team, including Rosa Bacchetta, Manuela Battaglia, Silvia Gregori, and Megan Levings.
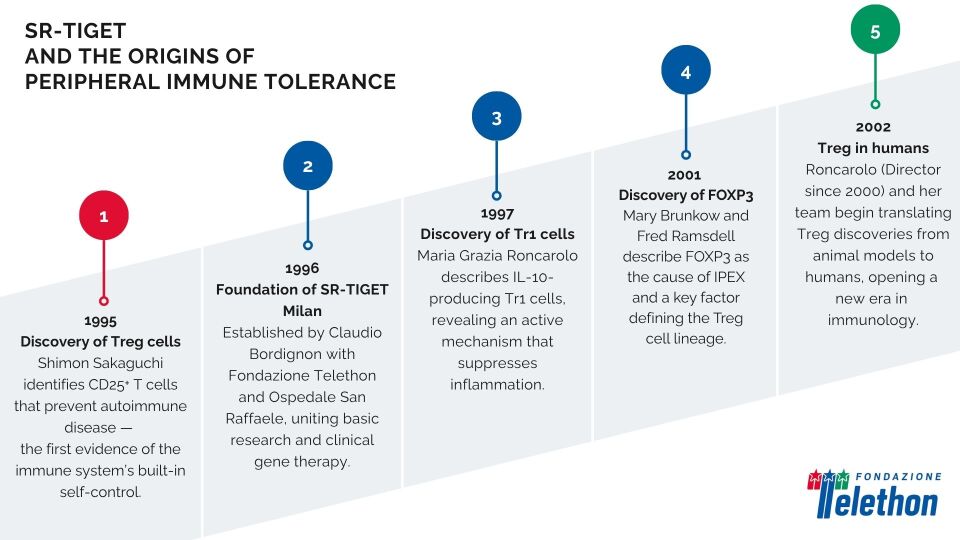
It all began in 1995, when Shimon Sakaguchi identified a subset of CD25⁺ T cells capable of preventing autoimmune diseases in mice — cells that would later become known as regulatory T cells (Tregs). These are a subset of T lymphocytes with a crucial role in keeping the immune system in check. Unlike the cells that attack viruses or bacteria, Tregs act as a kind of biological brake, preventing the immune system from turning against the body or overreacting to harmless stimuli.
Two years later, Maria Grazia Roncarolo, then at the DNAX Research Institute in California, published in Nature the first description of another class of regulatory T cells: the type-1 regulatory T cells (Tr1), characterized by the production of IL-10 – a molecule that helps dampen inflammation and limit excessive immune responses, thereby maintaining equilibrium and preventing autoimmune reactions.
These discoveries marked a conceptual turning point in immunology: peripheral tolerance is not a passive or secondary phenomenon, but an active process mediated by specialized cells essential for maintaining immune homeostasis and preventing self-reactivity. In other words, the human body possesses an intricate biological braking system that allows it to constantly distinguish between what must be attacked and what must be tolerated.
In 1998, Maria Grazia Roncarolo returned to Italy to join the newly founded SR-TIGET, established in 1996 by Fondazione Telethon and the Ospedale San Raffaele and directed by Claudio Bordignon. Two years later, she became the Director, further strengthening the translational strategy of the Institute, also with a focus on immunology.
Her laboratory quickly became an international reference point for the study of regulatory T cells and the mechanisms that control immune responses. The team – Rosa Bacchetta, Manuela Battaglia, Silvia Gregori and Megan Levings – expanded the pioneering work of Sakaguchi and Roncarolo, moving it from animal models to humans for the first time.
In 2002, together with Megan Levings, Roncarolo demonstrated that CD4⁺CD25⁺ T cells with the same suppressive properties described in mice are also present in humans. It was the first direct evidence of the existence and function of Treg cells in humans – a milestone that paved the way for a new era of human immunology.
The evidence of Treg cells in humans raised new questions: How are Tregs formed? Which genes control them? Could their therapeutic potential be harnessed in the lab?
The answers began to emerge in the early 2000s, with the identification of the FOXP3 gene and the discovery of its role in the autoimmune syndrome IPEX – another field where SR-TIGET played a decisive role.
FOXP3 acts as a true “master switch” of the immune system: it controls the development and function of Tregs, the cells responsible for maintaining the balance between defense and tolerance. When this gene is mutated, Tregs can no longer perform their regulatory role, and the immune system turns against the body itself. This is what happens in IPEX syndrome (Immunodysregulation Polyendocrinopathy Enteropathy X-linked), a rare and severe autoimmune disease affecting male infants. Children with IPEX develop intestinal inflammation, autoimmune diabetes and other immune disorders within the first months of life. The study of this condition has been crucial in revealing the link between FOXP3 and Tregs, also opening the door to innovative gene therapy strategies aimed at correcting the underlying defect.
At the same time, Roncarolo and Bacchetta also collaborated on studies focused on IPEX syndrome, a rare and severe autoimmune disease of early childhood. The turning point in understanding this disorder came in 2001, when Mary Brunkow and Fred Ramsdell identified mutations in the FOXP3 gene as the cause of IPEX. FOXP3 was then soon recognized as the master transcription factor defining the Treg cell lineage, first in mice and then in humans.
This genetic insight revolutionized the field, providing a biomarker that was instrumental in allowing scientists to precisely identify and isolate Tregs. Research on a rare disease thus proved essential to understanding a fundamental immune mechanism.
These studies marked the beginning of a new chapter in immunology, one in which basic research and translational medicine became increasingly intertwined. The discoveries of Roncarolo and her team not only redefined our understanding of immune tolerance but also laid the foundations for the development of the first cell- and gene-based therapies using regulatory T cells.
Thirty years on, the line of research that began in those years continues to evolve and inspire new generations of scientists. Today, at SR-TIGET, research on immune regulation and peripheral tolerance remains one of the institute’s scientific pillars: transforming fundamental discoveries into innovative therapeutic strategies for autoimmune and rare genetic diseases.
The insights born between 1995 and 2002 have forever changed the way we understand the immune system and its balance. These discoveries — from the identification of regulatory T cells to the decoding of the FOXP3 gene and its link with IPEX — have laid the scientific foundations on which today’s research into immune regulation and gene therapy continues to build.
Discover how these breakthroughs paved the way for the first gene therapies in the second chapter of this story, on line soon.
Further reading
- Sakaguchi S. et al., Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Journal of Immunology, 1995; 155(3):1151–1164.
- Groux H. et al., A CD4⁺ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature, 1997; 389:737–742.
- Levings M.K. et al., Human CD25⁺CD4⁺ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. Journal of Experimental Medicine, 2002; 196(10):1335–1346.
- Wildin R.S. et al., X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet, 2001 Jan;27(1):18-20.